What we do.
CPL specializes in developing, manufacturing, packaging, and testing non-sterile liquid and semi-solid pharmaceuticals. Our exclusive dedication to these forms has established us as industry frontrunners, allowing us to refine our expertise in each aspect.
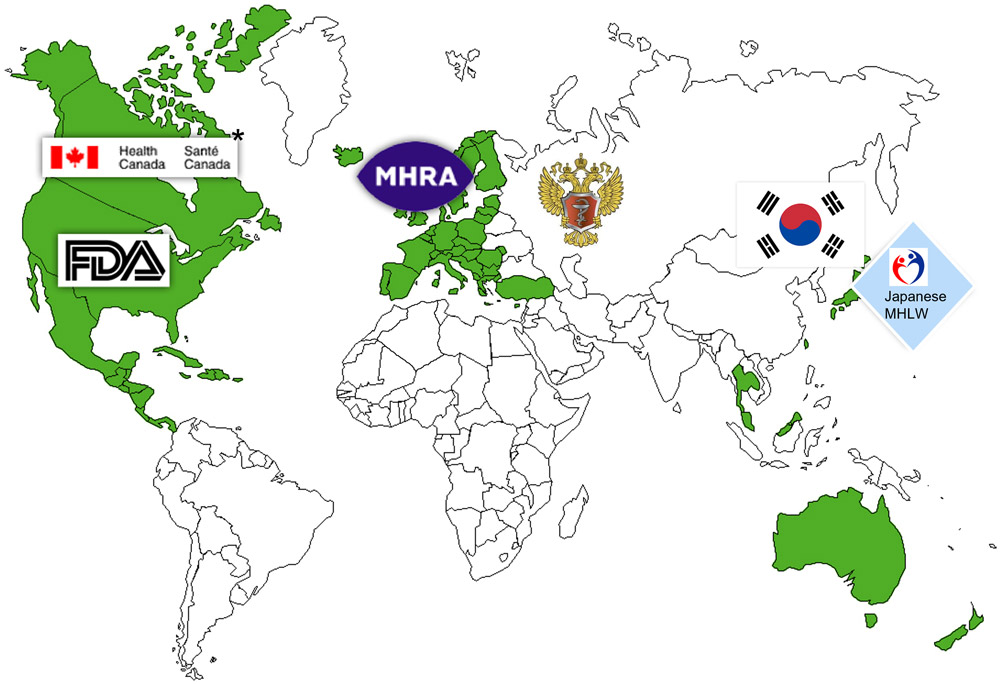
Global regulatory compliance and facility accreditation.
Our facilities hold registrations with the U.S. FDA and Health Canada and have undergone inspections by the Japanese Health Authority and Brazilian health agency. Additionally, a Mutual Recognition Agreement between Canada, the European Union, and Australia facilitates product releases to these regions. We undergo routine inspections by various international agencies, employing GMP or Pre-Approval Inspections. And our compliance team consistently conducts audits with both current and potential customers.
Product Development.
Our 35-member product development team boasts over 400 years of combined expertise in formulation development and analytics. We excel in striking the perfect balance between scientific rigor and efficient timelines.
Commercial manufacturing.
With our commercial manufacturing operations, we offer flexibility to accommodate diverse product requirements, all while delivering superior customer service.
Elemental Impurities Testing.
CPL offers elemental impurities testing services to current customers with development and/or commercial manufacturing programs and as a fee-for-service for companies who require testing. With state-of-the-art ICP-MS equipment and world-class analysts, CPL is capable of quantifying elements at the parts-per-billion level in compliance with USP <232> and <233>, EP, and ICH Q3D guidelines.
Skin Testing Services.
CPL’s Skin Lab provides cutting-edge in vitro release testing (IVRT), in vitro permeation testing (IVPT), and mass balance distribution analysis (MBDA) services. These are conducted in our GMP-certified environment, ensuring compliance with SUPAC-SS, USP <1724>, and USP <1090> standards.
