Let CPL be Your Guide to Regulatory Success in North America
Navigating the complex landscape of pharmaceutical regulatory affairs in the USA and Canada can be a daunting task. At CPL, we specialize in providing comprehensive regulatory affairs services tailored to the unique requirements of the FDA and Health Canada. Our expert team of regulatory professionals is dedicated to ensuring compliance, streamlining processes, and accelerating your path to market.
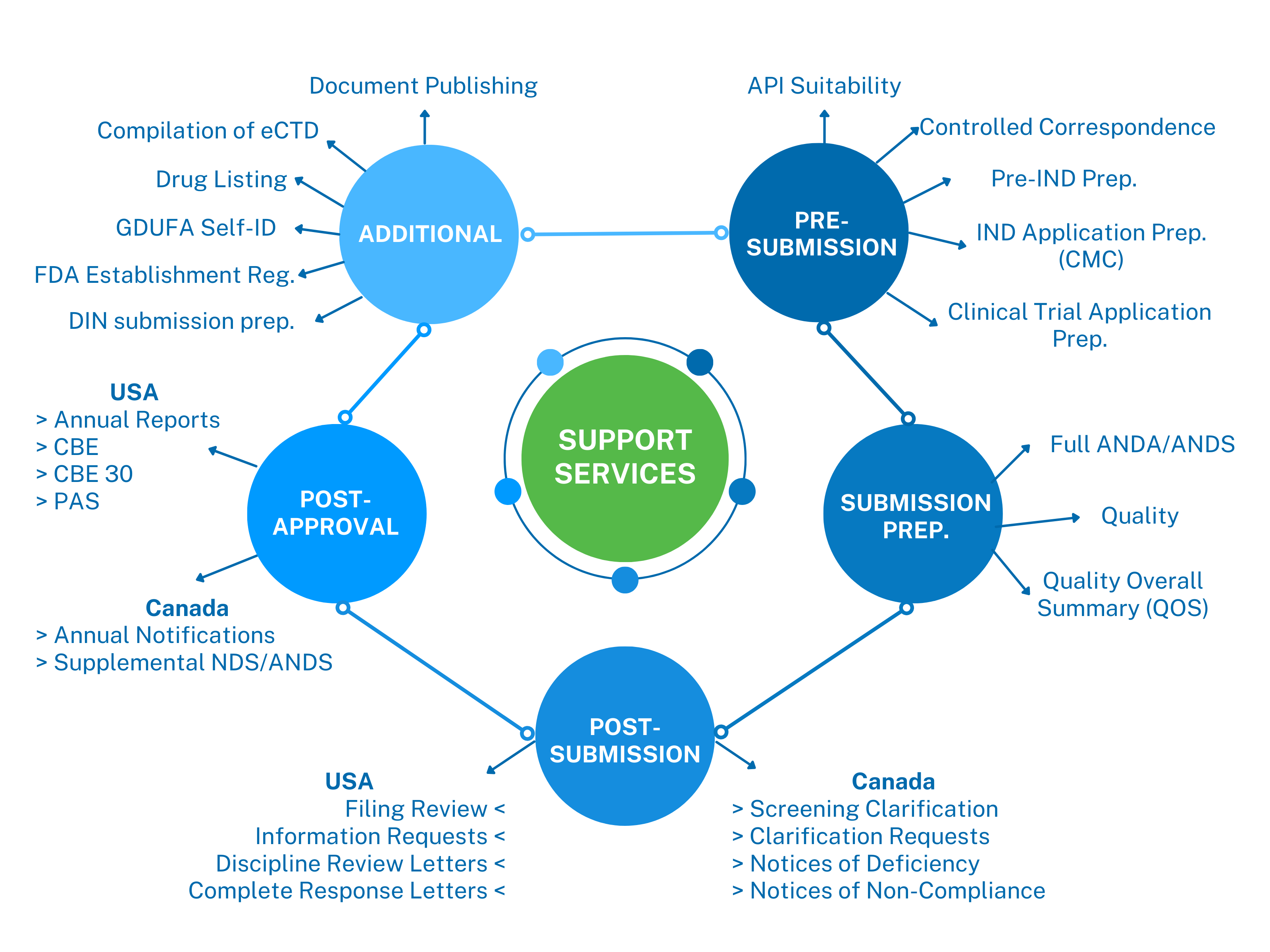
Scope of Services
We offer a wide range of regulatory affairs services tailored to meet the unique needs of pharmaceutical companies marketing their products in the USA and Canada. Our services include:
Why Choose CPL?
Extensive Regulatory Expertise
With years of experience in the pharmaceutical industry, our team possesses an in-depth understanding of the regulatory frameworks and requirements in both the USA (FDA) and Canada (Health Canada). We stay up-to-date with the latest regulations and guidelines to deliver accurate and timely advice.
Tailored Solutions
We recognize that every pharmaceutical product is unique, and our approach reflects this. Our regulatory affairs services are customized to meet your specific needs, whether you are a large pharmaceutical company or a small startup. We work closely with you to develop a regulatory strategy that aligns with your goals and maximizes your chances of success.
Efficient Market Entry
Time is of the essence in the pharmaceutical industry. Our regulatory experts meticulously guide you through the submission process, ensuring all CMC documentation is complete and compliant. By minimizing delays and addressing potential roadblocks proactively, we expedite your product’s entry into the US and Canadian markets.
Compliance and Risk Management
Regulatory compliance is crucial to maintaining a successful pharmaceutical business. At CPL, we help you navigate the intricate compliance requirements, mitigate risks, and address any regulatory challenges that may arise. Our proactive approach ensures your products remain in good standing throughout their lifecycle.
End-to-End Support
We offer comprehensive support throughout the regulatory affairs journey, from initial consultations to post-approval activities. Our services cover a wide range of regulatory activities, related to OTC and prescription drugs of various dosage forms.
Confidentiality and Security
We understand the importance of safeguarding your sensitive information. At CPL, we adhere to strict confidentiality protocols, ensuring that your proprietary data and regulatory strategies are protected at all times.
Hear from one of our experts
When it comes to navigating pharmaceutical regulatory affairs in North America, trust the experts at CPL. Our commitment to excellence, attention to detail, and unparalleled knowledge of the regulatory landscape make us the ideal partner to guide you through the complexities of compliance.
Get in touch
Contact us today to discuss your regulatory needs and navigate your product’s success in the USA and Canada. Together, we’ll bring your innovative and generic products to the market efficiently and responsibly.
